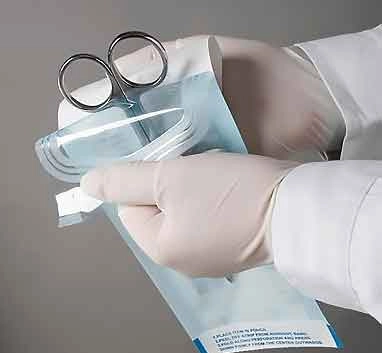
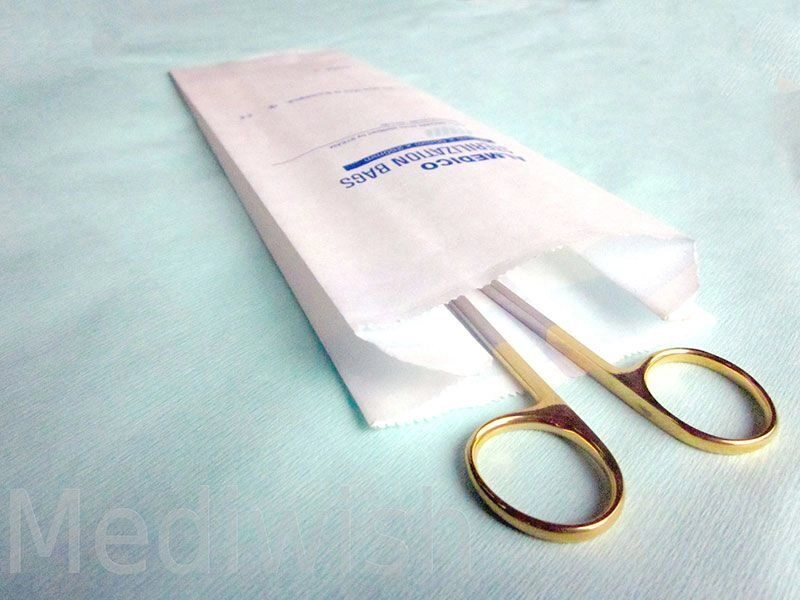
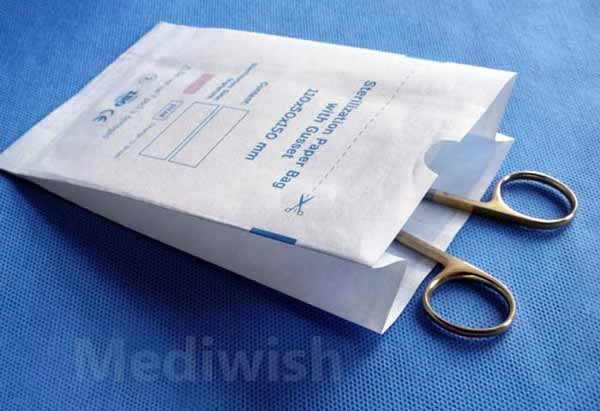
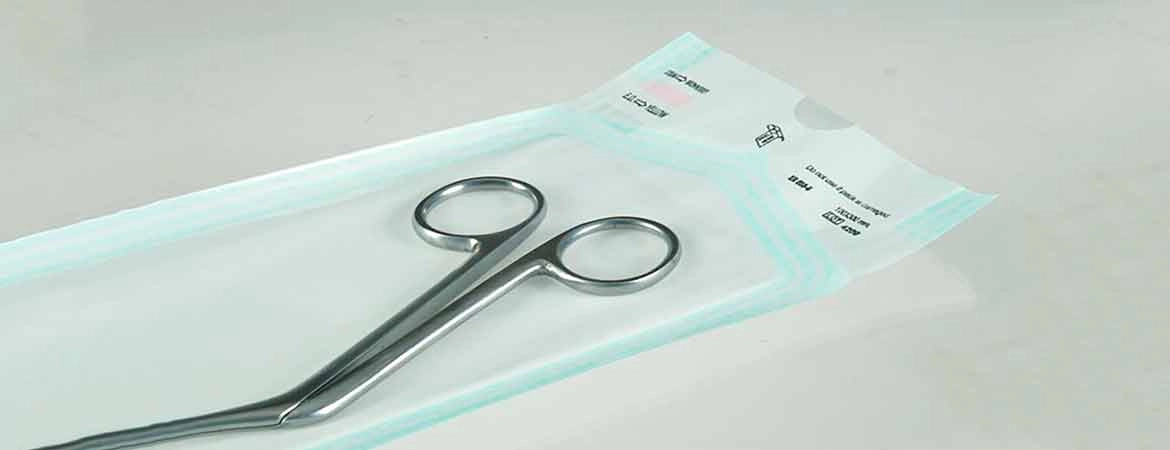
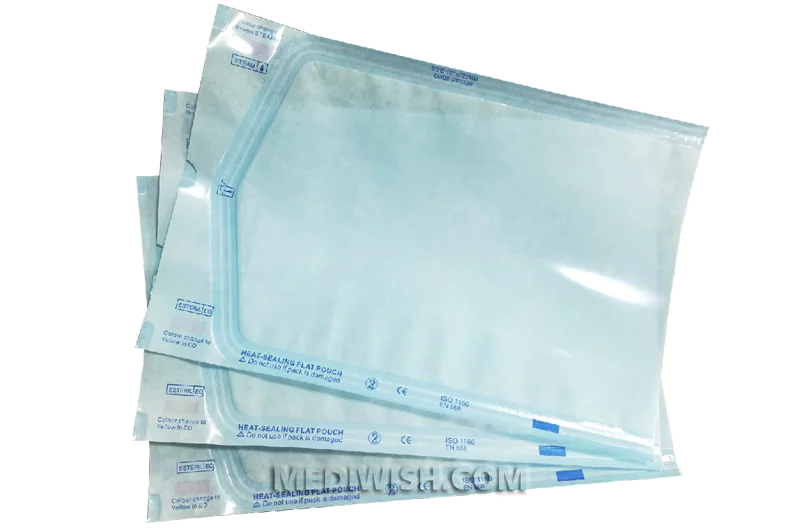
Self Sealing sterilization pouches for steam, ethylene oxide, steam-formaldehyde and radiation sterilization "Mediwish®" are easily permeable to the appropriate sterilizing agent, impervious to microorganisms when closed, and remain intact after sterilization by the appropriate method.
|
Product name
|
Self Seal Sterilization Pouch |
|
Country of Origin
|
China |
|
Instrument classification
|
Class I |
|
OEM
|
Available |
|
Properties
|
Medical Materials & Accessories |
|
Material
|
PE, medical grade paper+5.2s cpp.pet film |
|
Application area
|
Medical device packaging |
|
Usage
|
in Dental Office, Dental Labs, Medical Labs, Tatoo Instruments |
|
Intended sterilization type
|
Steam, Eto gas, Form |
|
Colour
|
clear / blue / green |
|
Quantity in box
|
200 pcs |
|
Packaging Details
|
carton size: 50*40*72cm |
|
Product Size
|
90*260mm |
|
Quality Certification
|
CE, ISO 13485 |
Mediwish Self Sealing Sterilization Pouches: Confidence in Sterility
Ensure patient safety and efficient instrument sterilization with Mediwish Self-Sealing Sterilization Pouches. These clinically proven pouches (made with medical-grade paper) are steam and ethylene oxide (EO) gas sterilization compatible, offer a reliable, cost-effective and safe solution for dental practices, tattoo studios, veterinary clinics, and various healthcare settings.
Applications:
- Dental Practices: Ideal for sterilizing dental instruments, burs, and other tools.
- Tattoo Studios: Ensures hygiene and sterility of needles, ink cups, and other equipment.
- Nail Salons: Maintains sterility of nail tools and implements used during procedures.
- Veterinary Clinics: Protects veterinary instruments and ensures aseptic technique during animal care.
Key Features:
- Medical-Grade Materials: Sterilization pouch self sealing Made with high-quality 60gsm French paper, complying with recognized material standards for exceptional durability and tear resistance.
- Universal Compatibility: Available in a range of self sealing sterilization pouch sizes (from 2.25" x 4", 3.5'' x 9'' to 7'' x 13") to accommodate various instruments.
- Effortless Sealing: Features a pre-folded design for fast and secure self-sealing without the need for a heat sealer, streamlining your workflow.
- Clear Sterilization Confirmation: Self seal sterilization pouch biuld with internal Class 1 chemical indicator change color (brown for steam, yellow for ETO) for visual verification of successful sterilization, ensuring sterile safety.
- Self-sealing pouches Latex free
- Autoclave pouch Easy storage efficiency
Self sealing sterilization pouch uses Benefits:
- Promotes Aseptic Technique: Provides a reliable barrier against microbial contamination, minimizing the risk of infections.
- Optimizes Workflow: Streamlines the steam sterilization process with a user-friendly design and efficient self-sealing closure.
- Cost-Effective Solution: Durable construction and bulk quantities (200 self sealing pouches per box; 4000/cs) offer long-lasting value.
- Safe Storage: Protects sterilized surgical instruments from dust and environmental contaminants until use, maintaining sterility.
- Efficient Sterilization: Facilitate a streamlined sterilization process with user-friendly self-sealing closure.
- Safe Storage: Maintain sterility of instruments with a reliable barrier against microbial contamination.
Ideal for: Dental practices, tattoo studios, nail salons, veterinary clinics, and more.
- Clear Content Recognition: Transparent CPP/PET film ensures easy identification of instruments.
- Universal Compatibility: Available in various sizes to accommodate different instrument needs.
Self-sealing sterilization pouches how to use:
- Place instruments inside the self seal pouch, ensuring they don't touch each other or the edges.
- Fold the adhesive strip following the pre-folded design for a secure seal.
- Visually inspect the self-sealing sterilization pouch for any tears or punctures before sterilization.
- Load sterilization pouches into the sterilizer according to the manufacturer's instructions.
- After sterilization, check the internal indicator for color change, confirming successful sterilization.
- Store sterilized pouches in a cool, dry environment until use.
Additional Tips:
- Do not overload autoclave pouches to ensure proper steam or gas penetration during sterilization.
- Dispose of used sterilizer pouches according to local regulations for biohazardous waste.
- Consult your sterilizer's user manual for specific recommendations on sterilization bag placement and cycle parameters.
Mediwish: Committed to Quality and Safety
At Mediwish, we prioritize providing healthcare professionals with reliable sterilization solutions that promote patient safety and efficient workflows. Our Self Sealing Sterilization Pouches are a testament to this commitment, offering a high-quality, user-friendly, and cost-effective option for instrument sterilization.
Order your box of Mediwish Self Seal Pouches today and experience the confidence of knowing your instruments are properly protected!
Additional Information:
- Certifications: CE, ISO 13485 and ISO 11140-1 for Chemical Indicators
- Shelf Life: 5 years from the date of manufacture (see instruction manual)
Flexible payment solutions for your convenience.
Bank Transfer:Secure and Reliable
Transfer funds directly to our company account. Request bank details, transfer funds, and notify us for seamless processing.
Cash Payment: Convenient and On-site
Pay in cash upon delivery. Inspect goods before payment. Check availability in your region.
Cashless Payments: Secure and Convenient
Pay electronically with Visa, MasterCard. Enter card details during checkout. Enjoy secure transactions.
Choose payment method that suits you best for a seamless purchasing experience.
Contact our customer service for assistance & inquiries.
Offer various to cater to your needs:
-In-house: (Hefei only): Warehouse can deliver order directly if you are in Hefei.
-Courier: (within China): Receive pack via courier service anywhere in China.
-Factory Pickup: Contact our employee with truck number to pick up your goods from us.
-International Postal Delivery: Choose EMS, FedEx, DHL, Usually Post. You'll got notification when your parcel arrives.
-Air Variant (packages > 20 kg): Opt: air cargo to nearest airport in your country.
-Multimodal Transportation: Enjoy container transport, rail, road options.
Shipments undergo official customs procedures, including customs export declaration.
Experience convenient forwarding with our versatile variations. Place order today & enjoy seamless delivery process.
Contract Manufacturing Hospital Sterilization Pouches and Reels for B2B International Customers
As the demand for hospital sterilization equipment continues to rise worldwide, the need for high-quality, reliable, and cost-effective sterilization pouches and reels has become more important than ever. Contract manufacturing of these critical products is an effective way for hospitals and other healthcare organizations to ensure that they have access to the latest technologies and materials while minimizing their own capital investments. This article will provide a detailed overview of contract manufacturing of hospital sterilization pouches and reels, with a focus on B2B international customers.