Cleaning validation is critical to medical device reprocessing, ensuring that contaminants like oils, tissues, soil, and microorganisms are effectively removed during cleaning. It is imperative to quantify the contaminant volume on reusable medical devices after undergoing cleaning processes.
The Significance of Cleaning Validation
Cleaning validation plays a pivotal role in maintaining patient safety and the effectiveness of reusable medical devices. During their intended use, medical devices are exposed to various contaminants, compromising their performance and putting patients at risk. Effective cleaning validation ensures that these contaminants are thoroughly removed, promoting the safe and reliable reuse of medical instruments.
The Role of Quantification in Cleaning Validation
Quantifying the volume of contaminants on reusable devices is a fundamental step in the cleaning validation process. By determining the microbial and soil load level, healthcare professionals can gauge the efficacy of the cleaning procedures. This quantification helps ensure that sterilization of the medical devices is consistently successful, minimizing the risk of infections and ensuring patient safety.
Ring to guidelines provided by regulatory authorities and manufacturers.
The Significance of Cleaning Validation
Cleaning validation plays a pivotal role in maintaining patient safety and the effectiveness of reusable medical devices. During their intended use, medical devices are exposed to various contaminants, compromising their performance and putting patients at risk. Effective cleaning validation ensures that these contaminants are thoroughly removed, promoting the safe and reliable reuse of medical instruments.
The Role of Quantification in Cleaning Validation
Quantifying the volume of contaminants on reusable devices is a fundamental step in the cleaning validation process. By determining the microbial and soil load level, healthcare professionals can gauge the efficacy of the cleaning procedures. This quantification helps ensure that sterilization of the medical devices is consistently successful, minimizing the risk of infections and ensuring patient safety.
Indicators for Successful Cleaning Validation
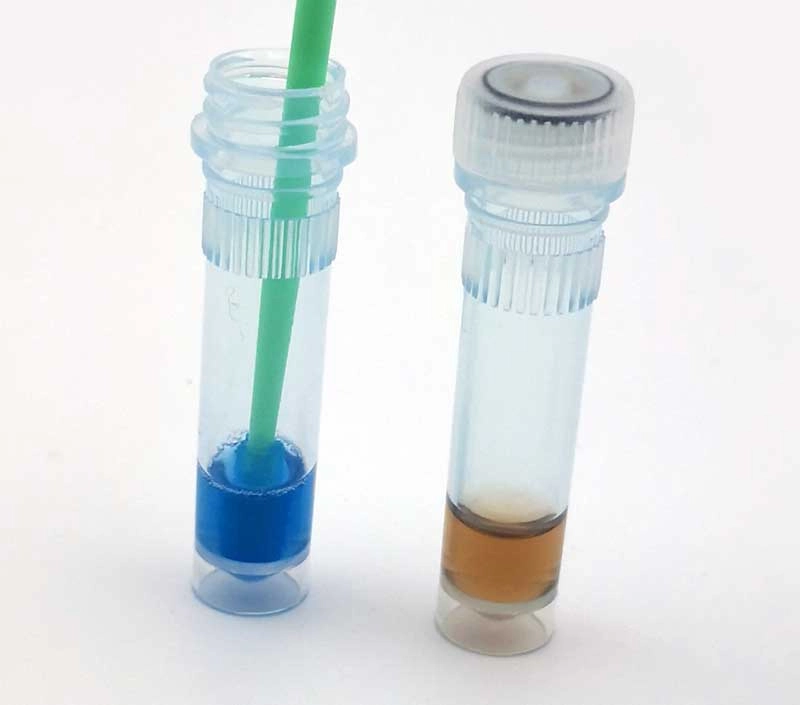
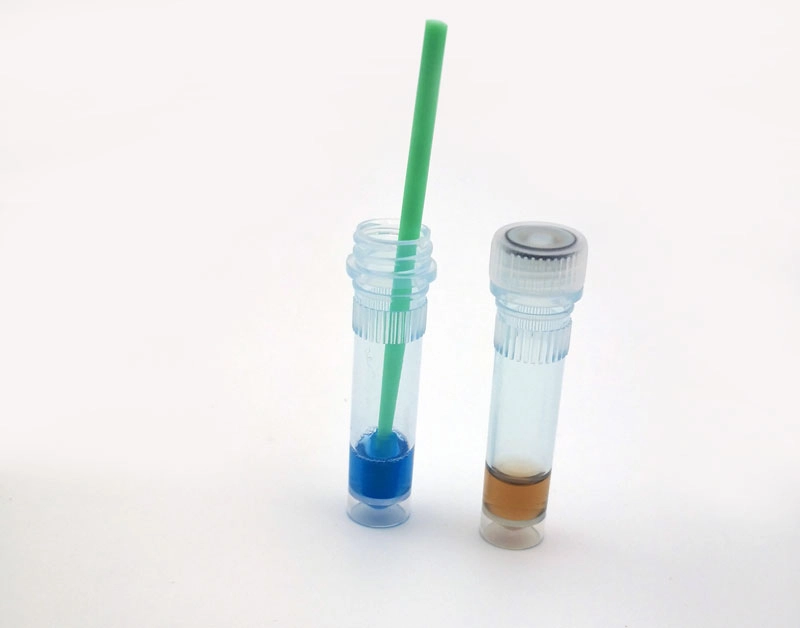
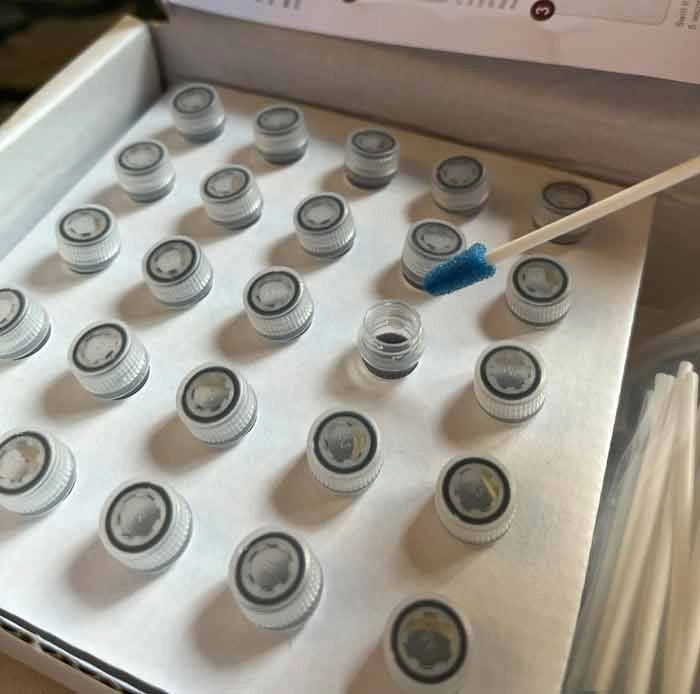
Protein Residue Test
The Protein Residue Test is a crucial indicator for assessing the effectiveness of cleaning procedures. It detects protein residues on medical instruments, which can harbor harmful microorganisms. This test helps identify areas that require further cleaning, enhancing the overall cleaning validation process.MEDIWISH™ Ultrasonic Indicator
Ultrasonic cleaning is widely used in medical device reprocessing. The MEDIWISH™ Ultrasonic Indicator is designed to validate the efficiency of ultrasonic cleaning machines. It ensures that the devices undergo thorough cleaning and are free from stubborn contaminants that might have adhered to their surfaces.Essential Wash Clean Indicators
These indicators offer a quick and reliable way to verify the success of the washing process. Essential Wash Clean Indicators use color change technology to indicate when medical instruments have been adequately cleaned. The visual cues these indicators provide simplify the validation process for healthcare professionals.STF Load Check Indicator
Sterilizer, Transport, and Function (STF) Load Check Indicator is a valuable tool for monitoring the efficiency of sterilization and transport processes. By utilizing this indicator, medical staff can ensure that medical devices reach the required level of cleanliness before they are used again.Soil Test for Washer Disinfectors
Washer disinfectors are commonly used to clean medical instruments. The Soil Test specifically assesses the cleaning efficacy of washer disinfectors. By using this indicator, healthcare facilities can verify whether their disinfection equipment is functioning optimally and providing the expected level of cleanliness.Ring to guidelines provided by regulatory authorities and manufacturers.