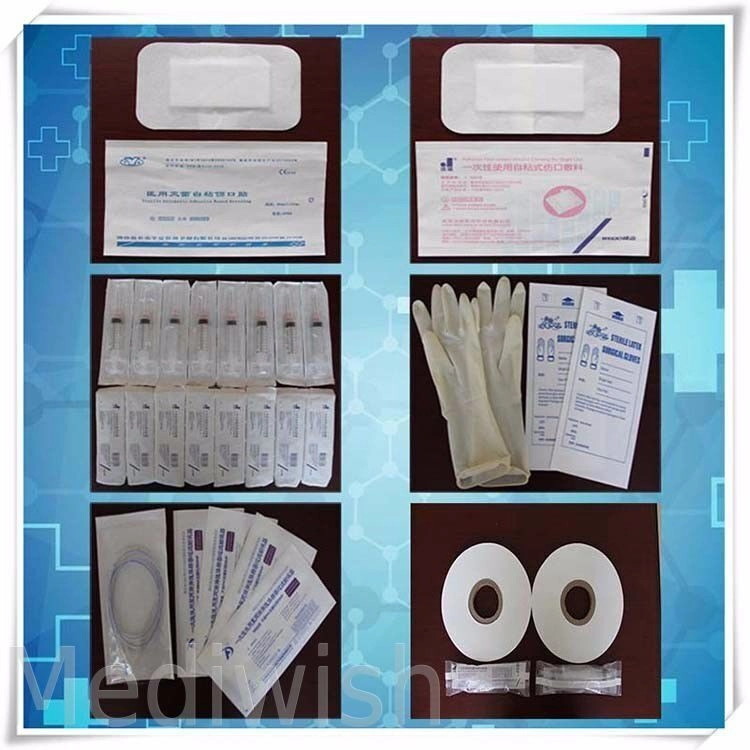
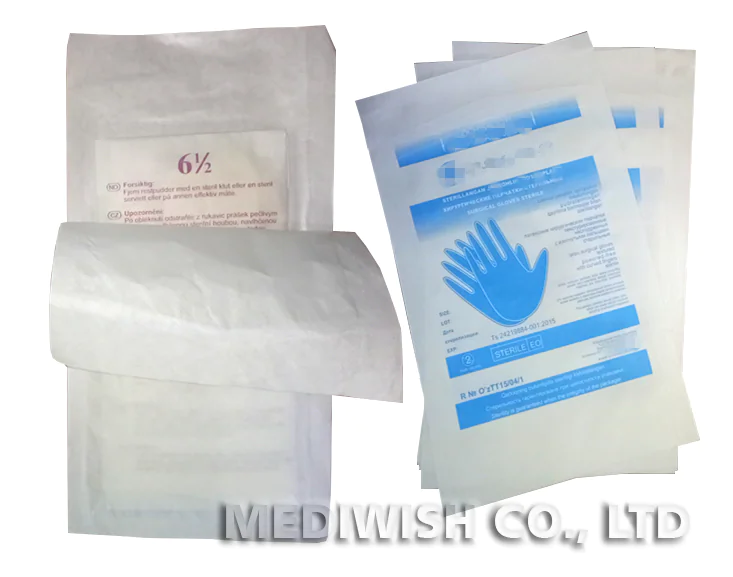
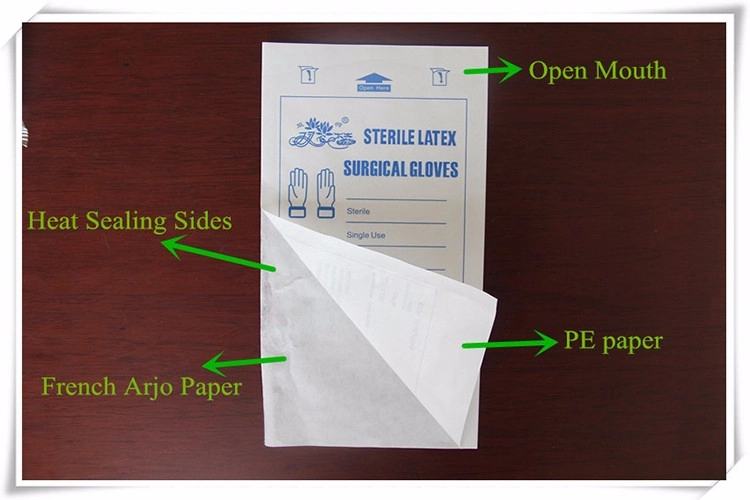
In the medical industry, ensuring the safety and effectiveness of medical equipment and supplies is of paramount importance. One crucial aspect of achieving this is through the use of proper sterilization techniques and packaging. Among the various methods available, ethylene oxide (ETO) sterilization has gained significant recognition for its ability to effectively sterilize medical products while maintaining their integrity. In this article, we will explore the significance of ETO sterilization packaging, the advantages it offers, and how it ensures safety and effectiveness in medical applications.
Introduction
Sterilization plays a vital role in preventing the transmission of infectious diseases and maintaining a sterile environment in healthcare settings. ETO sterilization is a widely used method due to its compatibility with various materials, including plastics, metals, and fabrics. To facilitate the sterilization process and maintain the sterility of medical products, ETO sterilization bags and pouches are utilized.
What is ETO Sterilization?
Ethylene oxide (ETO) sterilization is a low-temperature sterilization method that involves using a gas, ethylene oxide, to eliminate microorganisms from medical equipment and supplies. This process is particularly suitable for products that cannot withstand high-temperature sterilization methods such as autoclaving. ETO sterilization provides reliable and effective sterilization while preserving the physical and chemical properties of the sterilized items.
Importance of Sterile Packaging in the Medical Industry
Sterile packaging is crucial in the medical industry to maintain the sterility of medical devices, instruments, and supplies until they are ready for use. Improper packaging can compromise the integrity of the products and increase the risk of contamination. ETO sterilization packaging ensures that the items remain sterile throughout their shelf life, protecting patients from infections and healthcare providers from liability.
ETO Sterilization Packaging: An Overview
ETO sterilization packaging includes specialized bags and pouches designed to withstand the sterilization process and maintain sterility until the point of use. These packaging solutions are engineered to prevent the ingress of microorganisms while allowing the penetration of the sterilizing gas. They are available in various sizes and configurations to accommodate different types of medical products.
Advantages of ETO Sterilization Bags and Pouches
ETO sterilization bags and pouches offer several advantages that make them a preferred choice in the medical industry. Firstly, they provide an effective microbial barrier, preventing the entry of contaminants into the packaged items. Secondly, these packaging solutions are compatible with a wide range of materials, making them versatile for different medical applications. Additionally, ETO sterilization bags and pouches are durable, tear-resistant, and puncture-resistant, ensuring the integrity of the packaged items during handling and transportation.
Key Features of ETO Sterilization Packaging
ETO sterilization packaging possesses key features that contribute to its effectiveness and reliability. Firstly, these bags and pouches are constructed using medical-grade materials that meet stringent quality standards. They are designed to withstand the sterilization process, ensuring no compromise in performance. Additionally, ETO sterilization packaging incorporates proper sealing mechanisms to maintain the sterility of the packaged items.
How ETO Sterilization Bags Ensure Safety and Effectiveness
ETO sterilization bags are engineered to ensure the safety and effectiveness of the sterilization process. The bags are designed to allow the proper diffusion of ETO gas, ensuring thorough sterilization of the packaged items. Moreover, they provide an effective microbial barrier, preventing the recontamination of the sterilized products after the sterilization process is complete.
Choosing the Right ETO Sterilization Bags and Pouches
When selecting ETO sterilization bags and pouches, it is essential to consider the specific requirements of the medical application. Factors such as the size and shape of the items, the desired packaging configuration, and the compatibility with the sterilization equipment should be taken into account. Consulting with reputable manufacturers or suppliers can help in choosing the right packaging solutions that meet the unique needs of the medical facility.
Best Practices for Using ETO Sterilization Packaging
To ensure optimal performance and safety, certain best practices should be followed when using ETO sterilization packaging. It is crucial to properly load the bags or pouches, allowing adequate space for gas diffusion. The packaging should be securely sealed to maintain sterility, and proper labeling and documentation should accompany each package to facilitate traceability and compliance with regulatory standards.
Common Applications of ETO Sterilization Bags and Pouches
ETO sterilization bags and pouches find applications in various areas of the medical industry. They are commonly used for packaging surgical instruments, medical implants, implantable devices, and single-use medical supplies. These packaging solutions are also utilized in the pharmaceutical and biotechnology sectors for the sterilization and packaging of drug delivery systems, diagnostic kits, and laboratory equipment.
Sterilization Process Using ETO Sterilizer Bags
The sterilization process using ETO sterilizer bags involves several steps to ensure effective sterilization. Firstly, the items to be sterilized are loaded into the bags or pouches, taking care not to overcrowd the packaging. The bags are securely sealed to create an airtight environment. Subsequently, the packages are placed in the ETO sterilizer, where they undergo the sterilization cycle. Once the process is complete, the bags are aerated to remove any remaining traces of ETO gas before they can be safely used.
Compliance and Regulatory Standards for ETO Sterilization Packaging
The use of ETO sterilization bags and pouches is subject to compliance with regulatory standards and guidelines. Regulatory bodies such as the Food and Drug Administration (FDA) and International Organization for Standardization (ISO) have established requirements for sterilization processes and packaging materials. It is crucial for medical facilities to adhere to these standards to ensure patient safety and regulatory compliance.
Maintenance and Storage of ETO Sterilization Bags
Proper maintenance and storage of ETO sterilization bags are essential to ensure their effectiveness and longevity. These bags should be stored in a clean and dry environment, away from direct sunlight and heat sources. Regular inspections should be conducted to check for any signs of damage or degradation. Damaged or expired bags should be discarded, and new ones should be obtained to maintain the sterility of medical products.
Cost-Effectiveness of ETO Sterilization Packaging
Despite the initial investment, ETO sterilization packaging offers long-term cost-effectiveness. The ability to sterilize a wide range of materials and the durability of the packaging solutions contribute to their cost-effectiveness. Additionally, ETO sterilization bags and pouches help reduce the risk of product recalls and liability associated with contaminated medical products, leading to potential cost savings for healthcare facilities.
ETO sterilization packaging plays a crucial role in ensuring the safety and effectiveness of medical equipment and supplies. With its ability to sterilize a wide range of materials and maintain sterility throughout the shelf life of the packaged items, ETO sterilization bags and pouches provide a reliable solution for the medical industry. By following best practices, adhering to regulatory standards, and choosing the right packaging solutions, healthcare facilities can enhance patient safety and protect their reputation.
FAQs (Frequently Asked Questions)
Q1. What is the difference between ETO sterilization bags and pouches?
ETO sterilization pouches are generally larger and can accommodate larger medical equipment, while pouches are smaller and suitable for packing smaller items or tool kits. Specially designed for sterilization in an ethylene oxide atmosphere. Packages usually have additional inscriptions in the form of the logo of the manufacturer of the packaged goods, the name of the product, its characteristics, expiration date, production date, packaging date, date of sterilization.
Q2. Are ETO sterilization bags reusable?
No, ETO sterilization bags are typically designed for single-use only to ensure optimal sterility and prevent cross-contamination. In addition, they are coated with an ethylene oxide sterilization indicator, which irreversibly changes color when sterilized with ethylene oxide.
Q3. Can ETO sterilization bags be used with all types of medical equipment?
ETO sterilization bags are compatible with a wide range of materials, including plastics, metals, and fabrics, making them suitable for various types of medical equipment and supplies.
Q4. Are ETO sterilization bags environmentally friendly?
ETO sterilization bags are made from materials that are recyclable, contributing to environmental sustainability. Proper disposal methods should be followed to minimize their impact on the environment.
Q5. Where can I purchase ETO sterilization bags and pouches?
You can purchase ETO sterilization bags and pouches with your design from us. Mediwish is a sterilization pouches and roll manufacturer specializing in medical packaging solutions.